一、法规介绍
加拿大医疗器械由加拿大卫生部(Health Canada/HC)监管。加拿大医疗器械框架以Canada Medical Device Regulation(SOR/98-282/CMDR)为基础,同时又有指南文件对产品分类,产品注册,标签要求,官费支付等做了细致的规定和介绍。

二、器械分类
在加拿大,医疗器械被分为Class I, Class II, Class III和Class IV,风险等级逐步提高,Class I风险等级最低,Class II为中低风险,Class III为中高风险,而Class IV则代表最高风险。
I类医疗器械不受上市前审查的约束,但制造商必须建立并保持符合CMDR的证据并申请获得MDEL(Medical Device Establishment Licence)。II, III和IV类医疗器械必须接受上市前审查,通过MDL(Medical Device Licence)获得许可,无需申请MDEL,制造商必须建立并保持符合CMDR的证据。为了申请MDL,制造商必须持有MDSAP的认证。
三、上市路径
I类医疗器械的制造商仅要求获得MDEL证书,II, III和IV类医疗器械的经销商也进口商也是要求申请MDEL证书的。MDEL申请周期为120天,具体流程如下:
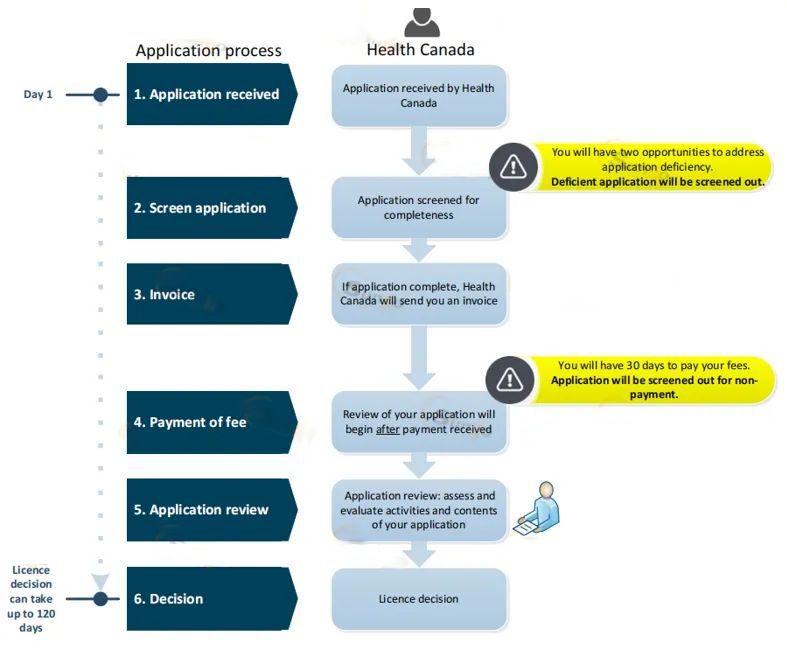
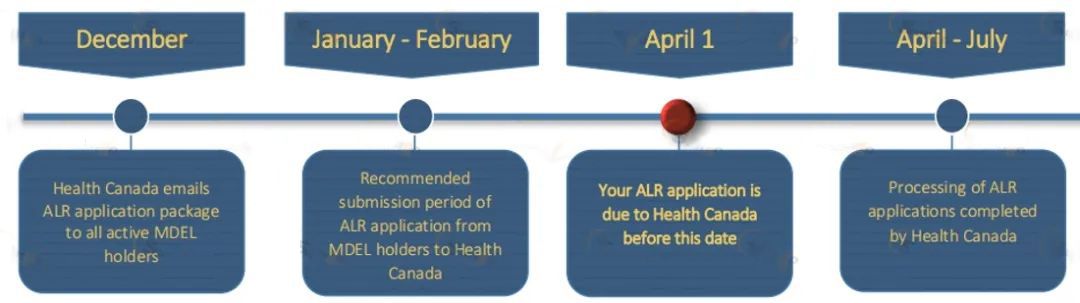
MDEL证书无有效期,需每年更新,Health Canada会在12月给MDEL持有者发送ALR(Annual Licence Review),持证方应在每年4月1日前提交其MDEL年度更新申请,具体流程见下图:
II,III 和IV类医疗器械上市需由制造商申请MDL证书,周期为2-10个月,不同风险等级的产品审批周期有所差异。其申请流程包括以下步骤:
步骤1:获得MDSAP证书
步骤2:准备MDL申请要求的技术文件
步骤3:缴纳MDL申请费用
步骤4:提交MDL注册申请
步骤5:加拿大官方审核 (包括行政审核,合规性审核,技术性审核)
步骤6:发布问题解决直至通过审批
步骤7:更新MDL注册并向加拿大官方支付年费
总而言之,MDEL是企业注册许可证,针对的是企业;而MDL则是医疗器械批准证书,针对的是产品。
MDL和MDEL的更新日期也有不同,MDEL续期的方式是在4月1日前提交年审申请并支付费用。MDL则要求在每年11月1日之前完成年度更新并支付费用。
MDEL证书:
MDL证书:

四、加拿大医疗器械申请问答
问:MDEL证书可以包含多少个产品?
答:MDEL证书是针对于企业,而非是器械。若仅生产一类医疗器械,可以只申请MDEL证书并完成年度更新即可;
问:我们公司有ISO 13485证书,是否可以替代MDSAP证书来申请MDL证书?
答:不可以。加拿大自2019年1月1日起,不再接受CMDCA颁发的CAN/CSA-ISO 13485:16证书,因此,现行所有II, III和IV类医疗器械的制造商必须切换到医疗器械单一审核程序(MDSAP);
问:我是贴牌商,无法申请体系证书该如何处理?
答:可以使用OEM的MDSAP证书。
五、FDASUNGO服务
FDASUNGO迄今已为多个客户成功申请了MDSAP证书,以及MDEL和MDL证书,产品包含了II类灭菌产品和III类有源器械。II类产品从提交申请到完成审批最快仅用2周!
FDASUNGO针对客户医疗器械产品出口加拿大的需求,提供全方位的服务项目:
MDSAP体系辅导;
MDEL证书申请;
MDL证书申请;
产品标签审核服务
产品检测服务;
加拿大上市后监督技术服务。
- 其他国家
- 暂无标签

